We had, some time ago, treated in an article of the “Amateurism of the ONSSA” on the occasion a file we had dealt with a little over three years ago.
A few weeks ago, an importer submitted his complaints to the ONSSA (National Office for Sanitary Safety of Food Products) following the blockage, and confirmation of refoulement, of its shipment of green tea imported from China. We will examine this file, received for expertise by my Cabinet, which will show us that instead of learning from their mistakes to progress like anyone else, ONSSA on the contrary seems amnesic and, as time goes by, sinks deeper into mediocrity.
After several unsuccessful approaches to the ONSSA in question, the company MARTEAPACK asked us, dated July 11, to assess if their imported green tea from China, actually blocked at the port of Agadir since April 27, is conforming to Moroccan law enforced by ONSSA
Document 1
We then wrote to ONSSA asking them to kindly inform us of the regulatory elements that support the blockage in question.
Document 2
The organization then sent MARTEAPACK a note (undated) on behalf of the Director General ONSSA, signed by Mr. Zakaria Abdelkader, Director of the Department of Control and Protection at ONSSA.
Document 3
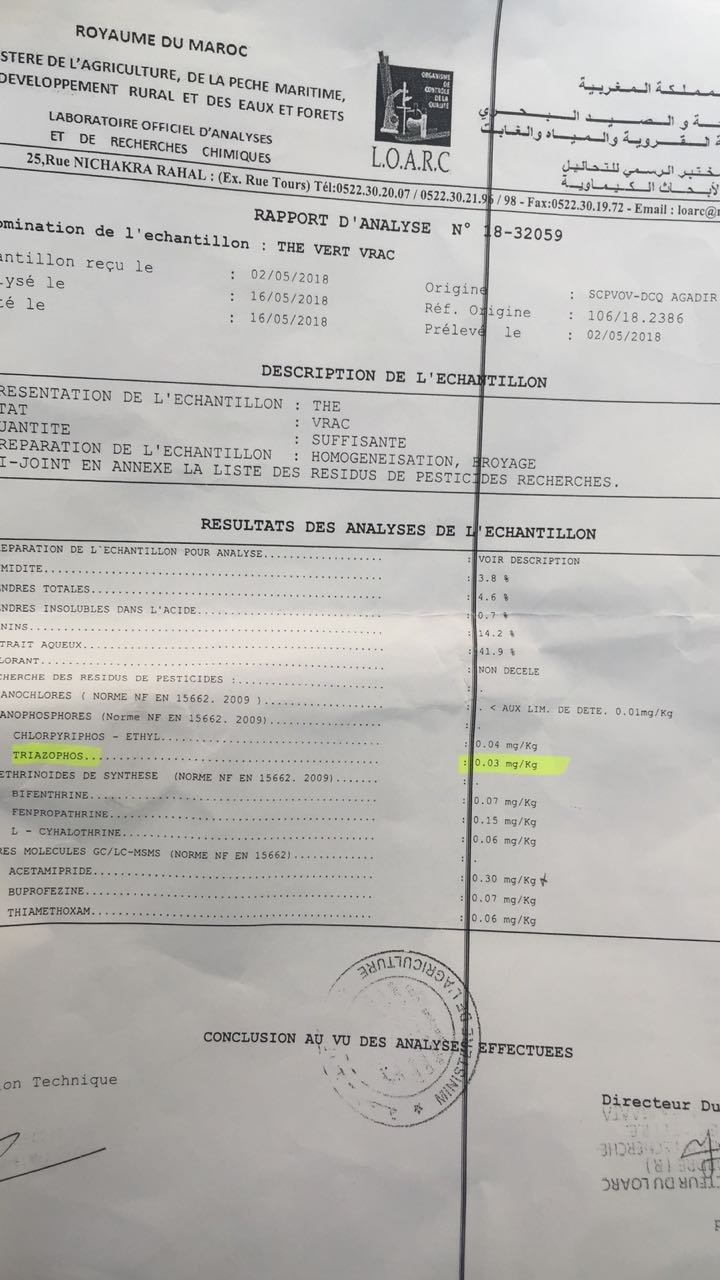
The note of the Director General, which refers to a request of MARTEAPACK of June 8th (see below under exegesis), states that the blockage is dictated by the presence in the tea of the insecticide “Triazophos” at the concentration of 0.03 mg / kg in a sample of the goods controlled by the LOARC (Official Laboratory for Analysis and Chemical Research) in Casablanca, but sampled in Agadir by an unidentified third party.
Document 4
At the same time, Mr. Zakaria’s note closed the door on a possible second check (contradictory expertise) requested by MARTEAPACK.
In parallel, and after a fortnight of waiting for a response to our note (Document 2), we made a reminder fax dated July 26th.
Document 5
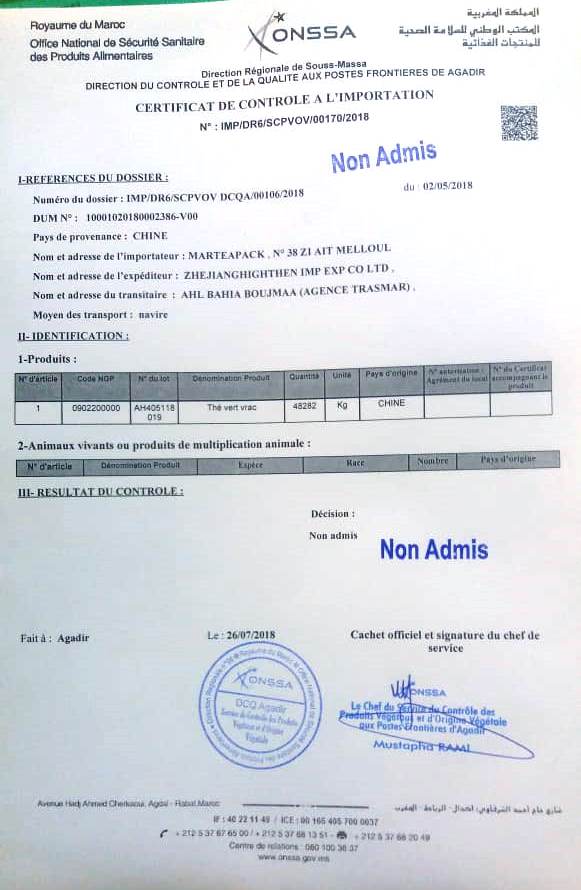
As a response, ONSSA sends (the same day) directly to MARTEAPACK a “Certificate of Control” carrying for all information a stamp of “Not Accepted” of the goods (see below under exegesis).
Document 6
We then write a warning note about ONSSA’s unorthodox practices that we hand-deliver to ONSSA and the Ministry of Agriculture as the Supervisory Authority.
Document 7
Document 8
The next day (July 27th), the customs services of Agadir called the forwarding agent of MARTEAPACK to inform him of refoulement action taken by ONSSA. Also, MARTEAPACK should inform Customs immediately of their preference for the incineration or reshipment of the “non-admitted” green tea batch. Distraught, the boss of the company calls me urgently for opinion. In our turn, we give our appreciation in a note on these unorthodox practices of ONSSA that we communicate to the President of MARTEAPACK for delivery to his lawyers. MARTEAPACK finally opted to hand over our note to ONSSA.
Document 9
Exegesis
The LOARC Analysis Bulletin
An analytical document that does not provide information on the method used for laboratory determinations, supported by appropriate and accepted scientific references, that does not give the degree of uncertainty of the measurements, not make a conclusion on the work done and does not show the name and the quality of the person who signs it, which seems to be the case of the truncated document of LOARC (Document 4) cannot correctly claim for qualification as Bulletin of Analyzes.
The maximum residue limit of Triazophos that would be accepted in the case of tea, or an assimilated food, is not included either.
So, for us, it is a document that does not live up to what is normally referred to as an Analysis Bulletin and, as a result, is not reliable to attest with confidence of the presence of Triazophos and / or the estimation of the concentration attributed to this insecticide in the lot of tea.
Note from the Director General ONSSA
The note in question (Document 3), which must have justified the “Certificate” of non-admission of the goods (Document 6), deserves a moment’s pause to recall that the advent of the law 28- 07 of food safety in 2010 was to be followed, 18 months after its entry into force, with the definitive repeal of Law 13-83 (previous law), including its chapter on the repression of fraud. Nevertheless (see our archives), the ONSSA still uses, a little à la carte, the chapter of the repression of the frauds of the repealed law 13-83 whenever this organization wishes it. But, while the chapter of the repression of the frauds, always in application, provides for the possibility of a second expertise to the applicant on his request, Mr. Zakaria answers no! (Document 3). To specify that the services of the ONSSA of Agadir refused to accuse (on June 8, 2018) reception of the note of request of MARTEAPACK for a second analysis, obliging the company to simply fill a printed form (see under object of Document 3), which was refused a copy of them as a discharge. To justify his refusal of a contradictory expert analysis, Mr. Zakaria argues that “the goods have been controlled according to the regulations in force“! We do not know if by speaking so ambiguously, Mr. Zakaria means that sometimes their services control but “non-regulatory”!
Also, knowing that the responsibility on the control is one and indivisible and that, moreover, the control of a cargo of such an importance (about fifty thousand kilos of tea) must relate to several samples distributed on all of this commodity (pre-established sampling plan) with determination in the analysis of an average and an experimental deviation not included in the LOARC Truncated Bulletin, the control to which ONSSA refers is, in our opinion, everything except regulatory and, in the absence of other details, it is therefore, for us, null and void.
On another level, it is worth recalling that since the introduction of the so-called “Delaney clause” in the United States Food and Drug Administration (FDA) regulation of 1958, which has inspired many countries around the world, it is accepted that if a chemical element is recognized as potentially carcinogenic, it must not be found, whatever the concentration, in any food whatsoever. The Triazophos must escape this rule since only its concentration (0.03mg / kg) is questioned (Document 3) by ONSSA. But in this case, we ignore the accepted threshold for estimating the degree of transgression of the Maximum Residue Limit (MRL) and Mr. Zakaria provides no information on this. This did not prevent his subordinate, Mr. Mustapha Rami ONSSA Agadir, decreeing the refoulement of the cargo of tea (Document 6) on the basis of that determination whereas we do not even know if a threshold value of the insecticide was actually exceeded in this commodity.
All this makes their mentioned «Certificate» simply a junk paper thousands far of light years from a responsible work done correctly.
At the time of writing this article, the national press echoes the revitalization of the free trade agreement signed between Morocco and the United States planned to enter into force in 2006 already. While His Majesty King Mohammed VI gave his instructions in his last Speech from the Throne for the Moroccan Administration to respond to professionals within a period of not more than one month, it is these ONSSA officials, experts proven Procrastination, who will be the contact partners of the FDA. In this respect, the FDA always, once it has blocked a commodity at the entrance of the US market, displays besides the blocking the reason set out in the law that motivated the blockage and invites the owner of the goods or his agent, to explain to the FDA.
Document 10
With the new Food Safety Modernization Act (FSMA), being implemented recently by FDA, the US has gone a long way in easing FDA administrative rules and, at the same time, empowering the private sector to streamline the flow of the food trade. This gives the private sector extra work. And, of course, this will require more time for operators that they will not be willing to sacrifice simply in trips to satisfy the extravagances of ONSSA as was the case for the managers of MARTEAPACK, and we too, with displacements between Dakhla, Agadir, Casablanca and Rabat. These kinds of movements do not benefit operators but take time, money, energy simply as a result of Procrastination and the poor work of ONSSA.
In this respect, the dialogue that needs to take place between our officials here and those of the FDA on the other side of the Atlantic will certainly be interesting to follow.